AbbVie (ABBV) Gets FDA Breakthrough Tag for Lung Cancer Drug
AbbVie ABBV announced that the FDA has granted a Breakthrough Therapy Designation (BTD) to its investigational antibody-drug conjugate (ADC) telisotuzumab vedotin (Teliso-V) for previously treated EGFR wild type non-small cell lung (NSCLC) cancer in patients with high levels of c-Met overexpression.
The Breakthrough Therapy status is granted to medicines being evaluated for serious conditions where early clinical evidence indicates the medicines’ potential for substantial improvement over available therapies on one or more clinically significant endpoints. At present, no cancer therapy is approved to treat patients with c-Met overexpressing NSCLC.
Teliso-V is being studied in a phase II LUMINOSITY study in patients with second- or third-line NSCLC with c-Met overexpression while a phase III study is expected to begin in the first half of 2022. The BTD designation was based on data from the LUMINOSITY study, which showed that the overall response rate (ORR) in the c-Met high group of patients with EGFR WT non-squamous NSCLC was 53.8%. ORR is the study’s primary endpoint. At a previously reported interim analysis, ORR in the c-Met intermediate group was 25%
AbbVie’s stock has risen 34.1% in the past year compared with an increase of 24.5% for the industry.
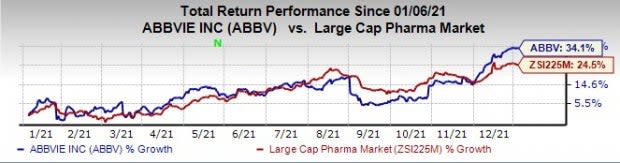
Image Source: Zacks Investment Research
Teliso-V is a key part of AbbVie’s oncology pipeline. AbbVie has built a substantial oncology franchise with Imbruvica and Venclexta. It expects oncology to be its major growth driver over the next 10 years. AbbVie markets Venclexta/Venclyxto in partnership with Roche RHHBY and Imbruvica with J&J JNJ.
AbbVie and Roche jointly commercialize Venclexta in the United States while AbbVie markets it outside the country.
Imbruvica, currently approved for five hematologic cancers, has multi-billion-dollar potential. Several studies on AbbVie and J&J’s Imbruvica are ongoing to evaluate the drug alone or in combination in different patient segments. AbbVie and J&J are positioning Imbruvica as a “pipeline in a molecule” - the treatment is in several company-sponsored studies.
AbbVie and Roche are also studying Venclyxto/Venclexta to expand the label to address the broader relapsed/refractory chronic lymphocytic leukemia (CLL) patient population, expand into earlier lines of therapy, and broaden into other hematologic malignancies like multiple myeloma and AML. Label expansion approvals in the past couple of years have expanded the eligible patient population of Venclexta significantly, which is boosting sales from the drug.
AbbVie currently has a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Another stock worth considering is Pfizer PFE, which has a Zacks Rank of 1 (Strong Buy). Pfizer’s stock has risen 53.7% in the past year.
Estimates for Pfizer’s 2022 earnings have gone up from $4.77 to $5.50 over the past 30 days.
Pfizer’s earnings performance has been mixed, with the company exceeding earnings expectations in three of the last four quarters while missing in one. PFE has a four-quarter earnings surprise of 10.85%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
